Abstract
In this interview, we spoke with Dr. Molly Accola of the University of Wisconsin. Dr. Accola holds a Masters in Medical Science from Harvard Medical School and a Ph.D. in Immunology from Harvard University, with a post-doctoral residency at Mayo Clinic. She worked as an R&D scientist at Third Wave Technologies, Inc. (now Hologic), and Pharmaceutical Product Development, LLC (PPD), both in Madison, WI. She has been the Senior Laboratory Development Specialist at the University of Wisconsin Hospital and Clinics since 2007, during which she has helped develop many assays. She has most recently started using next-generation sequencing to research potentially pathogenic variants in solid tumors.
Scientists have had a love-hate relationship with PCR amplification for decades. Real-time or quantitative PCR (qPCR) can be an amazingly powerful tool, but just like traditional PCR, it can be quite frustrating. There are several parameters that can influence the success of your PCR assay. We’ve highlighted ten things to consider when trying to improve your qPCR results.
There are a lot of choices when it comes to reverse transcriptases. Choosing the correct one for your cDNA synthesis and RT-PCR project is important. Here are a few questions that will lead you to right RT for your application:
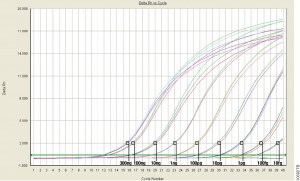
For those of us well versed in traditional, end-point PCR, wrapping our minds and methods around real-time or quantitative (qPCR) can be challenging. Here at Promega Connections, we are beginning a series of blogs designed to explain how qPCR works, things to consider when setting up and performing qPCR experiments, and what to look for in your results.
qPCR monitors amplification in real and allows you to measure starting material.
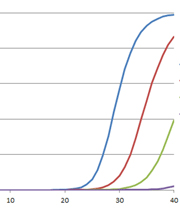
The polymerase chain reaction (PCR) has revolutionized modern biology as a quick and easy way to generate amazing amounts of genomic data. However, when PCR doesn’t work, it can be frustrating. At these times, PCR and reverse transcription PCR (RT-PCR) inhibitors seem to be everywhere: They lie dormant in your starting material and can co-purify with the template of interest, and they can be introduced during sample handling or reaction setup. The effects of these inhibitors can range from partial inhibition and underestimation of the target nucleic acid amount to complete amplification failure. What is a scientist to do?
The first step is education; you must learn what you’re up against. Here, I will focus on signs that inhibitors are causing problems with your quantitative PCR.

The first time I performed PCR was in 1992. I was finishing my Bachelors in Genetics and had an independent study project in a population genetics laboratory. My task was to try using a new technique, RAPD PCR, to distinguish clonal populations of the sea anemone, Metridium senile. These creatures can reproduce both sexually and asexually, which can make population genetics studies challenging. My professor was looking for a relatively simple method to identify individuals who were genetically identical (i.e., potential clones).

Working with RNA can be a tricky thing…it falls apart easily, and RNases (enzymes that degrade RNA) are ubiquitous. Successfully isolating RNA and maintaining its integrity is critical, especially when sensitive downstream applications are used (e.g., RNA-Seq).
Good techniques for RNA handling are simple to employ but crucial for success. All RNA purification and handling should take place in an RNase-free, RNA-only zone of the lab. Segregating RNA work from protein and DNA purification and handling will help minimize the potential for RNase contamination and help keep your RNA intact. Only buffer and water stocks treated to be RNase-free should be kept in the RNA area of the lab, and gloves should be worn at all times to prevent accidental contamination. Tools and equipment such as pipets, tips, and centrifuges should be designated for use only in the RNA zone as well. The location of the RNA zone in the lab is also important. Keeping traffic to a minimum and moving the RNA zone away from doors, windows, and vents can also help minimize contamination.
Back in graduate school, I purified a lot of RNA, and after a while, I became fairly successful at it. My yields were good, and the RNA was intact. However, many of my early attempts at RNA isolation yielded degraded RNA that did not work well in many downstream applications. In my case, successfully isolating high-quality RNA required practice. During my trials and tribulations, I learned a lot of tricks and tips about how to obtain high-quality RNA. Here I share some of these tricks to help you speed through that “practice makes perfect” phase so that you can isolate RNA like a pro.
First, a disclaimer: Even though I learned a lot during my time in the lab, I’m not saying that I was a pro when it came to RNA isolation. After months of “practice”, my RNA looked really good but never quite as good as that of one of the PostDocs in our lab, and I have to admit that I experienced an occasional twinge of jealousy. Even if you never reach RNA purification perfection, the information presented here should help you improve your RNA isolation results.
Today’s guest blog was written in collaboration with Melissa Martin, a former global marketing intern with Promega. She is a senior at the University of Wisconsin-Madison where she is double majoring in zoology and life sciences communication, with a certificate in environmental studies.

Peer-reviewed papers are considered the most technical and in-depth way to learn about research and scientific advances. As a student or scientist, you will not only want to read scholarly articles to learn about what others are doing in your field but also to expand your knowledge and learn about scientific advances in completely new areas of study. With countless disciplines of science covering wide-ranging topics such as cell biology, physical chemistry or human behavior, it can be overwhelming to do a general search and find articles and journals that will have the topics relevant to your interests.

Q: What is the easiest way to clone PCR Products?
A: The simplest way to clone PCR Products is to amplify the product using thermostable polymerases such as Taq, Tfl or Tth polymerase. These polymerases add a single deoxyadenosine to the 3´-end of the amplified products (3´-end overhang), and can be cloned directly into a linearized T-vector.
Today’s guest blog was written in collaboration with Melissa Martin, a former global marketing intern with Promega. She is a senior at the University of Wisconsin-Madison where she is double majoring in zoology and life sciences communication, with a certificate in environmental studies.
Have you ever found yourself wondering what the newest advancements were on genetically engineering plants or using artificial intelligence in biotechnology but didn’t know where to start looking? You most likely know the basic science behind the headlines, but a general web search may lead to dramatized articles that focus more on getting attention than being accurate. Or you might find a scholarly article that will offer in-depth, peer-reviewed information but may require more time to read than you are willing to give.

From macrophages that seek out and destroy infectious agents to fibroblasts that hold tissues and organs together, cells give form and function to our bodies. However, despite their foundational roles in our biology, there is still much we don’t know about cells—like where different cell types are localized, what states a given cell type may take on, how the molecular characteristics of cells change over a person’s lifetime and more. Addressing these questions will provide a deeper understanding about the cellular and genetic basis of human health and disease.